Battery Storage Bank
How long do solar batteries last?
There are two ways to answer this question and the first is to determine how long a solar battery can power your home. In many cases, a fully charged battery can run your home overnight when your solar panels are not producing energy. To make a more exact calculation, you'll need to know a few variables, including how much energy your household consumes in a given day, what the capacity and power rating is for your solar battery and whether or not you are connected to the electric grid.
For the sake of a simple example, we'll determine the size of a battery needed to provide an adequate solar plus storage solution with national average data from the U.S. Energy Information Administration. The average U.S. household will use roughly 30 kilowatt-hours (kWh) of energy per day and a typical solar battery can deliver some 10 kWh of capacity. Thus a very simple answer would be, if you purchased three solar batteries, you could run your home for an entire day with nothing but battery support.
In reality, the answer is more complicated than that. You will also be generating power with your solar panel system during the day which will offer strong power for some 6-7 hours of the day during peak sunlight hours. On the other end, most batteries cannot run at maximum capacity and generally peak at a 90% DoD (as explained above). As a result, your 10 kWh battery likely has a useful capacity of 9 kWh.
Ultimately, if you are pairing your battery with a solar PV array, one or two batteries can provide sufficient power during nighttime when your panels are not producing. However, without a renewable energy solution, you may need 3 batteries or more to power your entire home for 24 hours. Additionally, if you are installing home energy storage in order to disconnect from the electric grid, you should install a few days' worth of backup power to account for days where you might have cloudy weather.
Solar battery lifespan
The general range for a solar battery's useful lifespan is between 5 and 15 years. If you install a solar battery today, you will likely need to replace it at least once to match the 25 to 30 year lifespan of your PV system. However, just as the lifespan of solar panels has increased significantly in the past decade, it is expected that solar batteries will follow suit as the market for energy storage solutions grows.
Proper maintenance can also have a significant effect on your solar battery's lifespan. Solar batteries are significantly impacted by temperature, so protecting your battery from freezing or sweltering temperatures can increase its useful life. When a PV battery drops below 30° F, it will require more voltage to reach maximum charge; when that same battery rises above the 90° F threshold, it will become overheated and require a reduction in charge. To solve this problem, many leading battery manufacturers, like Tesla, provide temperature moderation as a feature. However, if the battery that you buy does not, you will need to consider other solutions like earth-sheltered enclosures. Quality maintenance efforts can definitely impact how long your solar battery will last.
What are the best batteries for solar?
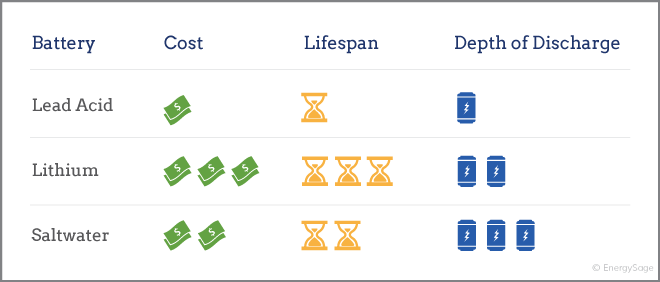
Batteries used in home energy storage typically are made with one of three chemical compositions: lead acid, lithium ion, and saltwater. In most cases, lithium ion batteries are the best option for a solar panel system, though other battery types can be more affordable.
-
Lead acid
Lead acid batteries are a tested technology that has been used in off-grid energy systems for decades. While they have a relatively short life and lower DoD than other battery types, they are also one of the least expensive options currently on the market in the home energy storage sector. For homeowners who want to go off the grid and need to install lots of energy storage, lead acid can be a good option.
-
Lithium
The majority of new home energy storage technologies, such as the , use some form of lithium ion chemical composition. Lithium ion batteries are lighter and more compact than lead acid batteries. They also have a higher DoD and longer lifespan when compared to lead acid batteries. However, lithium ion batteries are more expensive than their lead acid counterparts.
-
Saltwater
A newcomer in the home energy storage industry is the saltwater battery. Unlike other home energy storage options, saltwater batteries don’t contain heavy metals, relying instead on saltwater electrolytes. While batteries that use heavy metals, including lead acid and lithium ion batteries, need to be disposed of with special processes, a saltwater battery can be easily recycled. However, as a new technology, saltwater batteries are relatively untested, and the one company that makes solar batteries for home use (Aquion) filed for bankruptcy in 2017.
Types of Lithium-ion
Become familiar with the many different types of lithium-ion batteries.
Lithium-ion is named for its active materials; the words are either written in full or shortened by their chemical symbols. A series of letters and numbers strung together can be hard to remember and even harder to pronounce, and battery chemistries are also identified in abbreviated letters.
For example, lithium cobalt oxide, one of the most common Li-ions, has the chemical symbols LiCoO2 and the abbreviation LCO. For reasons of simplicity, the short form Li-cobalt can also be used for this battery. Cobalt is the main active material that gives this battery character. Other Li-ion chemistries are given similar short-form names. This section lists six of the most common Li-ions. All readings are average estimates at time of writing.
Lithium Cobalt Oxide(LiCoO2) — LCO
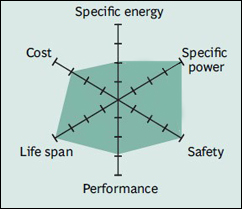
Its high specific energy makes Li-cobalt the popular choice for mobile phones, laptops and digital cameras. The battery consists of a cobalt oxide cathode and a graphite carbon anode. The cathode has a layered structure and during discharge, lithium ions move from the anode to the cathode. The flow reverses on charge. The drawback of Li-cobalt is a relatively short life span, low thermal stability and limited load capabilities (specific power). Figure 1 illustrates the structure.
|
.jpg)
|
Figure 1: Li-cobalt structure.
The cathode has a layered structure. During discharge the lithium ions move from the anode to the cathode; on charge the flow is from cathode to anode.
Source: Cadex |
The drawback of Li-cobalt is a relatively short life span, low thermal stability and limited load capabilities (specific power). Like other cobalt-blended Li-ion, Li-cobalt has a graphite anode that limits the cycle life by a changing solid electrolyte interface (SEI), thickening on the anode and lithium plating while fast charging and charging at low temperature. Newer systems include nickel, manganese and/or aluminum to improve longevity, loading capabilities and cost.
Li-cobalt should not be charged and discharged at a current higher than its C-rating. This means that an 18650 cell with 2,400mAh can only be charged and discharged at 2,400mA. Forcing a fast charge or applying a load higher than 2,400mA causes overheating and undue stress. For optimal fast charge, the manufacturer recommends a C-rate of 0.8C or about 2,000mA. The mandatory battery protection circuit limits the charge and discharge rate to a safe level of about 1C for the Energy Cell.
The hexagonal spider graphic (Figure 2) summarizes the performance of Li-cobalt in terms of specific energy or capacity that relates to runtime; specific power or the ability to deliver high current; safety; performance at hot and cold temperatures; life span reflecting cycle life and longevity; and cost. Other characteristics of interest not shown in the spider webs are toxicity, fast-charge capabilities, self-discharge and shelf life. The Octagon Battery – What makes a Battery a Battery).
The Li-cobalt is losing favor to Li-manganese, but especially NMC and NCA because of the high cost of cobalt and improved performance by blending with other active cathode materials. (See description of the NMC and NCA below.)
|
.jpg)
|
Figure 2: Snapshot of an average Li-cobalt battery.
Li-cobalt excels on high specific energy but offers only moderate performance specific power, safety and life span.
Source: Cadex |
Summary Table
Lithium Cobalt Oxide: LiCoO2 cathode (~60% Co), graphite anode
Short form: LCO or Li-cobalt. Since 1991 |
| Voltages |
3.60V nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) |
150–200Wh/kg. Specialty cells provide up to 240Wh/kg. |
| Charge (C-rate) |
0.7–1C, charges to 4.20V (most cells); 3h charge typical. Charge current above 1C shortens battery life. |
| Discharge (C-rate) |
1C; 2.50V cut off. Discharge current above 1C shortens battery life. |
| Cycle life |
500–1000, related to depth of discharge, load, temperature |
| Thermal runaway |
150°C (302°F). Full charge promotes thermal runaway |
| Applications |
Mobile phones, tablets, laptops, cameras |
Comments
2019 update: |
Very high specific energy, limited specific power. Cobalt is expensive. Serves as Energy Cell. Market share has stabilized.
Early version; no longer relevant. |
Table 3: Characteristics of lithium cobalt oxide.
Lithium Manganese Oxide (LiMn2O4) — LMO
Li-ion with manganese spinel was first published in the Materials Research Bulletin in 1983. In 1996, Moli Energy commercialized a Li-ion cell with lithium manganese oxide as cathode material. The architecture forms a three-dimensional spinel structure that improves ion flow on the electrode, which results in lower internal resistance and improved current handling. A further advantage of spinel is high thermal stability and enhanced safety, but the cycle and calendar life are limited.
Low internal cell resistance enables fast charging and high-current discharging. In an 18650 package, Li-manganese can be discharged at currents of 20–30A with moderate heat buildup. It is also possible to apply one-second load pulses of up to 50A. A continuous high load at this current would cause heat buildup and the cell temperature cannot exceed 80°C (176°F). Li-manganese is used for power tools, medical instruments, as well as hybrid and electric vehicles.
Figure 4 illustrates the formation of a three-dimensional crystalline framework on the cathode of a Li-manganese battery. This spinel structure, which is usually composed of diamond shapes connected into a lattice, appears after initial formation.
|
.jpg)
|
Figure 4: Li-manganese structure.
The cathode crystalline formation of lithium manganese oxide has a three-dimensional framework structure that appears after initial formation. Spinel provides low resistance but has a more moderate specific energy than cobalt.
Source: Cadex |
Li-manganese has a capacity that is roughly one-third lower than Li-cobalt. Design flexibility allows engineers to maximize the battery for either optimal longevity (life span), maximum load current (specific power) or high capacity (specific energy). For example, the long-life version in the 18650 cell has a moderate capacity of only 1,100mAh; the high-capacity version is 1,500mAh.
Figure 5 shows the spider web of a typical Li-manganese battery. The characteristics appear marginal but newer designs have improved in terms of specific power, safety and life span. Pure Li-manganese batteries are no longer common today; they may only be used for special applications.
|
.jpg)
|
Figure 5: Snapshot of a pure Li-manganese battery.
Although moderate in overall performance, newer designs of Li-manganese offer improvements in specific power, safety and life span.
Source: Boston Consulting Group |
Most Li-manganese batteries blend with lithium nickel manganese cobalt oxide (NMC) to improve the specific energy and prolong the life span. This combination brings out the best in each system, and the LMO (NMC) is chosen for most electric vehicles, such as the Nissan Leaf, Chevy Volt and BMW i3. The LMO part of the battery, which can be about 30 percent, provides high current boost on acceleration; the NMC part gives the long driving range.
Li-ion research gravitates heavily towards combining Li-manganese with cobalt, nickel, manganese and/or aluminum as active cathode material. In some architecture, a small amount of silicon is added to the anode. This provides a 25 percent capacity boost; however, the gain is commonly connected with a shorter cycle life as silicon grows and shrinks with charge and discharge, causing mechanical stress.
These three active metals, as well as the silicon enhancement can conveniently be chosen to enhance the specific energy (capacity), specific power (load capability) or longevity. While consumer batteries go for high capacity, industrial applications require battery systems that have good loading capabilities, deliver a long life and provide safe and dependable service.
Summary Table
Lithium Manganese Oxide: LiMn2O4 cathode. graphite anode
Short form: LMO or Li-manganese (spinel structure) Since 1996 |
| Voltages |
3.70V (3.80V) nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) |
100–150Wh/kg |
| Charge (C-rate) |
0.7–1C typical, 3C maximum, charges to 4.20V (most cells) |
| Discharge (C-rate) |
1C; 10C possible with some cells, 30C pulse (5s), 2.50V cut-off |
| Cycle life |
300–700 (related to depth of discharge, temperature) |
| Thermal runaway |
250°C (482°F) typical. High charge promotes thermal runaway |
| Applications |
Power tools, medical devices, electric powertrains |
Comments
2019 update: |
High power but less capacity; safer than Li-cobalt; commonly mixed with NMC to improve performance.
Less relevant now; limited growth potential. |
Table 6: Characteristics of Lithium Manganese Oxide.
Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
One of the most successful Li-ion systems is a cathode combination of nickel-manganese-cobalt (NMC). Similar to Li-manganese, these systems can be tailored to serve as Energy Cells or Power Cells. For example, NMC in an 18650 cell for moderate load condition has a capacity of about 2,800mAh and can deliver 4A to 5A; NMC in the same cell optimized for specific power has a capacity of only about 2,000mAh but delivers a continuous discharge current of 20A. A silicon-based anode will go to 4,000mAh and higher but at reduced loading capability and shorter cycle life. Silicon added to graphite has the drawback that the anode grows and shrinks with charge and discharge, making the cell mechanically unstable.
The secret of NMC lies in combining nickel and manganese. An analogy of this is table salt in which the main ingredients, sodium and chloride, are toxic on their own but mixing them serves as seasoning salt and food preserver. Nickel is known for its high specific energy but poor stability; manganese has the benefit of forming a spinel structure to achieve low internal resistance but offers a low specific energy. Combining the metals enhances each other strengths.
NMC is the battery of choice for power tools, e-bikes and other electric powertrains. The cathode combination is typically one-third nickel, one-third manganese and one-third cobalt, also known as 1-1-1. This offers a unique blend that also lowers the raw material cost due to reduced cobalt content. Another successful combination is NCM with 5 parts nickel, 3 parts cobalt and 2 parts manganese (5-3-2). Other combinations using various amounts of cathode materials are possible.
Battery manufacturers move away from cobalt systems toward nickel cathodes because of the high cost of cobalt. Nickel-based systems have higher energy density, lower cost, and longer cycle life than the cobalt-based cells but they have a slightly lower voltage.
New electrolytes and additives enable charging to 4.4V/cell and higher to boost capacity. Figure 7 demonstrates the characteristics of the NMC.
|
.jpg)
|
Figure 7: Snapshot of NMC.
NMC has good overall performance and excels on specific energy. This battery is the preferred candidate for the electric vehicle and has the lowest self-heating rate.
Source: Boston Consulting Group |
There is a move towards NMC-blended Li-ion as the system can be built economically and it achieves a good performance. The three active materials of nickel, manganese and cobalt can easily be blended to suit a wide range of applications for automotive and energy storage systems (EES) that need frequent cycling. The NMC family is growing in its diversity.
Summary Table
Lithium Nickel Manganese Cobalt Oxide: LiNiMnCoO2. cathode, graphite anode
Short form: NMC (NCM, CMN, CNM, MNC, MCN similar with different metal combinations) Since 2008 |
| Voltages |
3.60V, 3.70V nominal; typical operating range 3.0–4.2V/cell, or higher |
| Specific energy (capacity) |
150–220Wh/kg |
| Charge (C-rate) |
0.7–1C, charges to 4.20V, some go to 4.30V; 3h charge typical. Charge current above 1C shortens battery life. |
| Discharge (C-rate) |
1C; 2C possible on some cells; 2.50V cut-off |
| Cycle life |
1000–2000 (related to depth of discharge, temperature) |
| Thermal runaway |
210°C (410°F) typical. High charge promotes thermal runaway |
| Cost |
~$420 per kWh (Source: RWTH, Aachen) |
| Applications |
E-bikes, medical devices, EVs, industrial |
Comments
2019 update: |
Provides high capacity and high power. Serves as Hybrid Cell. Favorite chemistry for many uses; market share is increasing.
Leading system; dominant cathode chemistry. |
Table 8: Characteristics of lithium nickel manganese cobalt oxide (NMC).
Lithium Iron Phosphate(LiFePO4) — LFP
In 1996, the University of Texas (and other contributors) discovered phosphate as cathode material for rechargeable lithium batteries. Li-phosphate offers good electrochemical performance with low resistance. This is made possible with nano-scale phosphate cathode material. The key benefits are high current rating and long cycle life, besides good thermal stability, enhanced safety and tolerance if abused.
Li-phosphate is more tolerant to full charge conditions and is less stressed than other lithium-ion systems if kept at high voltage for a prolonged time. How to Prolong Lithium-based Batteries). As a trade-off, its lower nominal voltage of 3.2V/cell reduces the specific energy below that of cobalt-blended lithium-ion. With most batteries, cold temperature reduces performance and elevated storage temperature shortens the service life, and Li-phosphate is no exception. Li-phosphate has a higher self-discharge than other Li-ion batteries, which can cause balancing issues with aging. This can be mitigated by buying high quality cells and/or using sophisticated control electronics, both of which increase the cost of the pack. Cleanliness in manufacturing is of importance for longevity. There is no tolerance for moisture, lest the battery will only deliver 50 cycles. Figure 9
summarizes the attributes of Li-phosphate.
Li-phosphate is often used to replace the lead acid starter battery. Four cells in series produce 12.80V, a similar voltage to six 2V lead acid cells in series. Vehicles charge lead acid to 14.40V (2.40V/cell) and maintain a topping charge. Topping charge is applied to maintain full charge level and prevent sulfation on lead acid batteries.
With four Li-phosphate cells in series, each cell tops at 3.60V, which is the correct full-charge voltage. At this point, the charge should be disconnected but the topping charge continues while driving. Li-phosphate is tolerant to some overcharge; however, keeping the voltage at 14.40V for a prolonged time, as most vehicles do on a long road trip, could stress Li-phosphate. Time will tell how durable Li-Phosphate will be as a lead acid replacement with a regular vehicle charging system. Cold temperature also reduces performance of Li-ion and this could affect the cranking ability in extreme cases.
|
|
Figure 9: Snapshot of a typical Li-phosphate battery.
Li-phosphate has excellent safety and long life span but moderate specific energy and elevated self-discharge.
Source: Cadex |
Summary Table
Lithium Iron Phosphate: LiFePO4 cathode, graphite anode
Short form: LFP or Li-phosphate Since 1996 |
| Voltages |
3.20, 3.30V nominal; typical operating range 2.5–3.65V/cell |
| Specific energy (capacity) |
90–120Wh/kg |
| Charge (C-rate) |
1C typical, charges to 3.65V; 3h charge time typical |
| Discharge (C-rate) |
1C, 25C on some cells; 40A pulse (2s); 2.50V cut-off (lower that 2V causes damage) |
| Cycle life |
2000 and higher (related to depth of discharge, temperature) |
| Thermal runaway |
270°C (518°F) Very safe battery even if fully charged |
| Cost |
~$580 per kWh (Source: RWTH, Aachen) |
| Applications |
Portable and stationary needing high load currents and endurance |
Comments
2019 update: |
Very flat voltage discharge curve but low capacity. One of safest
Li-ions. Used for special markets. Elevated self-discharge.
Used primarily for energy storage, moderate growth. |
Table 10: Characteristics of lithium iron phosphate.
Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
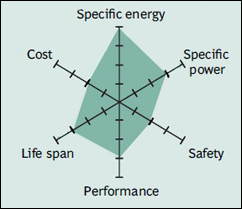
Lithium nickel cobalt aluminum oxide battery, or NCA, has been around since 1999 for special applications. It shares similarities with NMC by offering high specific energy, reasonably good specific power and a long life span. Less flattering are safety and cost. Figure 11 summarizes the six key characteristics. NCA is a further development of lithium nickel oxide; adding aluminum gives the chemistry greater stability.
|
|
Figure 11: Snapshot of NCA.
High energy and power densities, as well as good life span, make NCA a candidate for EV powertrains. High cost and marginal safety are negatives.
Source: Cadex |
Summary Table
Lithium Nickel Cobalt Aluminum Oxide: LiNiCoAlO2 cathode (~9% Co), graphite anode
Short form: NCA or Li-aluminum. Since 1999 |
| Voltages |
3.60V nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) |
200-260Wh/kg; 300Wh/kg predictable |
| Charge (C-rate) |
0.7C, charges to 4.20V (most cells), 3h charge typical, fast charge possible with some cells |
| Discharge (C-rate) |
1C typical; 3.00V cut-off; high discharge rate shortens battery life |
| Cycle life |
500 (related to depth of discharge, temperature) |
| Thermal runaway |
150°C (302°F) typical, High charge promotes thermal runaway |
| Cost |
~$350 per kWh (Source: RWTH, Aachen) |
| Applications |
Medical devices, industrial, electric powertrain (Tesla) |
Comments
2019 update: |
Shares similarities with Li-cobalt. Serves as Energy Cell.
Mainly used by Panasonic and Tesla; growth potential. |
Table 12: Characteristics of Lithium Nickel Cobalt Aluminum Oxide.
Lithium Titanate (Li2TiO3) — LTO
Batteries with lithium titanate anodes have been known since the 1980s. Li-titanate replaces the graphite in the anode of a typical lithium-ion battery and the material forms into a spinel structure. The cathode can be lithium manganese oxide or NMC. Li-titanate has a nominal cell voltage of 2.40V, can be fast charged and delivers a high discharge current of 10C, or 10 times the rated capacity. The cycle count is said to be higher than that of a regular Li-ion. Li-titanate is safe, has excellent low-temperature discharge characteristics and obtains a capacity of 80 percent at –30°C (–22°F).
LTO (commonly Li4Ti5O12) has advantages over the conventional cobalt-blended Li-ion with graphite anode by attaining zero-strain property, no SEI film formation and no lithium plating when fast charging and charging at low temperature. Thermal stability under high temperature is also better than other Li-ion systems; however, the battery is expensive. At only 65Wh/kg, the specific energy is low, rivalling that of NiCd. Li-titanate charges to 2.80V/cell, and the end of discharge is 1.80V/cell. Figure 13 illustrates the characteristics of the Li-titanate battery. Typical uses are electric powertrains, UPS and solar-powered street lighting.
|
.jpg)
|
Figure 13: Snapshot of Li-titanate.
Li-titanate excels in safety, low-temperature performance and life span. Efforts are being made to improve the specific energy and lower cost.
Source: Boston Consulting Group |
Summary Table
Lithium Titanate: Cathode can be lithium manganese oxide or NMC; Li2TiO3 (titanate) anode
Short form: LTO or Li-titanate Commercially available since about 2008. |
| Voltages |
2.40V nominal; typical operating range 1.8–2.85V/cell |
| Specific energy (capacity) |
50–80Wh/kg |
| Charge (C-rate) |
1C typical; 5C maximum, charges to 2.85V |
| Discharge (C-rate) |
10C possible, 30C 5s pulse; 1.80V cut-off on LCO/LTO |
| Cycle life |
7,000–30,000 |
| Thermal runaway |
One of safest Li-ion batteries |
| Cost |
~$1,005 per kWh (Source: RWTH, Aachen) |
| Applications |
UPS, electric powertrain (Mitsubishi i-MiEV, Honda Fit EV),
solar-powered street lighting |
Comments
2019 update: |
Long life, fast charge, wide temperature range but low specific energy and expensive. Among safest Li-ion batteries.
Ability to ultra-fast charge; high cost limits to special application. |
Table 14: Characteristics of lithium titanate.
Solid-state Li-ion: High specific energy but poor loading and safety.
Lithium-sulfur: High specific energy but poor cycle life and poor loading
Lithium-air: High specific energy but poor loading, needs clean air to breath and has short life.
Figure 15 compares the specific energy of lead-, nickel- and lithium-based systems. While Li-aluminum (NCA) is the clear winner by storing more capacity than other systems, this only applies to specific energy. In terms of specific power and thermal stability, Li-manganese (LMO) and Li-phosphate (LFP) are superior. Li-titanate (LTO) may have low capacity but this chemistry outlives most other batteries in terms of life span and also has the best cold temperature performance. Moving towards the electric powertrain, safety and cycle life will gain dominance over capacity. (LCO stands for Li-cobalt, the original Li-ion.)
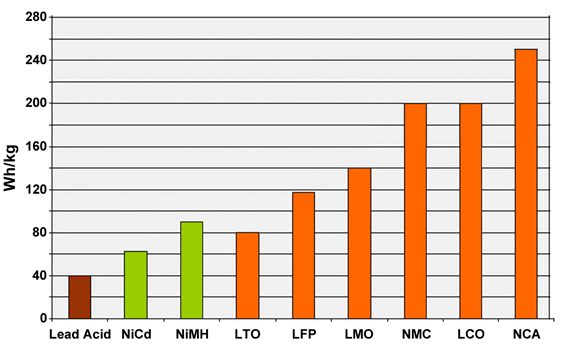
Figure 15: Typical specific energy of lead-, nickel- and lithium-based batteries.
NCA enjoys the highest specific energy; however, manganese and phosphate are superior in terms of specific power and thermal stability. Li-titanate has the best life span.